Brain Computer Interface
First Neuralink Brain-Computer Interface Implanted in a Person
In-human implantation of Neuralink's brain-computer interface is a milestone.
Updated February 1, 2024 Reviewed by Kaja Perina
Neuralink has implanted its brain-computer interface in a human, according to an announcement made on Monday by South African-born American billionaire entrepreneur Elon Musk, who co-founded the privately-held neurotechnology company with Max Hodak and others in 2016.
While this is a first for Neuralink, it is not the first time in science history that a human has been implanted with an invasive brain-computer interface (BCI), also known as brain-machine interfaces (BMI) in the brain.
Over two-and-a-half decades prior, in 1998, Roy E. Bakay, M.D., a neurosurgeon at Emory University, and neuroscientist Phillip R. Kennedy, M.D. implanted a neurotrophic electrode in the motor cortex of a Emory University Hospital patient with Lou Gehrig's disease, also known as amyotrophic lateral sclerosis (ALS), and another patient who was a brainstem stroke survivor. The brain-computer interface enabled both patients to communicate by using their thoughts to control a cursor to activate icons that prompted a computer to say phrases.
BCI providers include Synchron, Medtronic, Kernel, BrainGate, EMOTIV, NeuroSky, Neurable, NextMind (now part of Snap Labs), the University Medical Centre (UMC) in Utrecht, Netherlands, Ripple Neuro, OpenBCI, CGX (A Cognionics Company), Compumedics Neuroscan, ANT Neuro, NIRx Medical Technologies, LLC, Natus Medical Incorporated, g.tec medical engineering GmbH, Neuroelectrics, Nihon Kohden Corporation, Integra Lifesciences, Cadwell Industries, Inc., Cortech Solutions, Inc., and others.
Brain-computer interfaces (BCIs), also known as brain-machine interfaces (BMIs), are neurotechnology that enables a person to control external devices with thoughts of intended actions. Typically, brain-computer interfaces use artificial intelligence (AI) machine learning to help decode the massive amounts of complex brain activity data recordings. It is an industry that is projected to reach USD 6.2 billion by 2030, growing at a compound annual growth rate (CAGR) of 17.5% during 2023-2030 according to Grand View Research.
The BCIs are assistive technology that may improve the lives of those who are paralyzed or disabled. It is neurotechnology that offers a glimmer of hope to those who are severely impaired or have lost the ability to move or communicate. With brain-computer interfaces, a person can use their thoughts of intended action to control a wheelchair, robotic limbs, computer cursor, keyboard, communication devices, and more electronics that may improve the quality of daily life for those impacted by spinal cord injuries, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), stroke, and more.
“The first human received an implant from @Neuralink yesterday and is recovering well,” Musk posted on his social media platform X (formerly known as Twitter). “Initial results show promising neuron spike detection.”
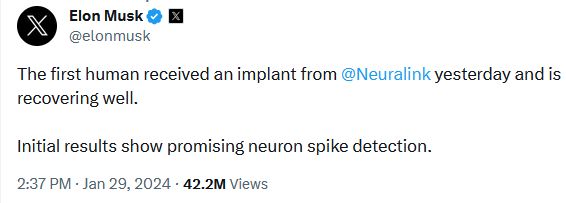
This represents a significant step forward for Neuralink’s Precise Robotically Implanted Brain-Computer Interface (PRIME) investigational medical device clinical trial for its brain-computer interface (BCI).
Last May, Neuralink was awarded by the U.S. Food and Drug Administration (FDA) with an investigational device exemption (IDE), which enables the company to conduct a clinical study to gather information on the safety and effectiveness of their investigational device. The Neuralink clinical trial will evaluate their brain-computer interface implant called the N1 Implant, the surgical robot called R1 Robot, and their N1 User App.
The N1 Implant is sealed airtight in a biocompatible enclosure with a battery that can be charged wirelessly, low-power chips, and 1024 electrodes spread over 64 threads. R1 Robot uses five camera systems, optics to conduct optical coherence tomography (OCT), and a needle thinner than human hair in order to insert the threads of the N1 Implant in the targeted brain location.
According to the company, the PRIME Study will span roughly six years from start to finish and involve nine clinic and at-home visits for an estimated year and a half, plus 20 long-term follow-up visits over half a decade.
Tetraplegia, better known as quadriplegia, is the partial or full paralysis where there is a loss of movement and sensation in both arms and legs. The target participants for Neuralink’s PRIME Study are tetraplegic (quadriplegic) adults who are 22 years-old or over with limited function in both arms and legs, due to amyotrophic lateral sclerosis (ALS) or cervical spinal cord injury from at least a year prior without improvement.
According to Neuralink, their mission is to “create a generalized brain interface to restore autonomy to those with unmet medical needs today and unlock human potential tomorrow,” and their initial aim for their BCI is to “to grant people the ability to control a computer cursor or keyboard using their thoughts alone.” With the first in-human implantation of their brain-computer interface, Neuralink and Musk have taken an important step forward towards achieving their vision of the future.
Copyright © 2024 Cami Rosso All rights reserved.




